Abstract
Recently, Chimeric Antigen Receptor (CAR)-T cell therapies show excellent therapeutic effect against various hematologic malignancies, including B-cell leukemia, B-cell lymphoma, and multiple myeloma. Expansion of target diseases of this attractive immunotherapy is one of the most important issues. To identify the novel specific tumor antigen as CAR-T cell target is the standard way to accomplish this goal. However, recent CAR-T cells recognize single tumor antigen, so normal tissues expressing the target antigen are possible to be attacked by CAR-T cells. For example, anti-CD19 CAR-T cells against B-cell malignancies kill not only tumor cells but also normal B-cells, so patients receiving the CAR-T cells showed severe immune deficiency. Improvement of target cell recognition system of CAR-T cells minimizes this adverse effect and contributes to expand target diseases.
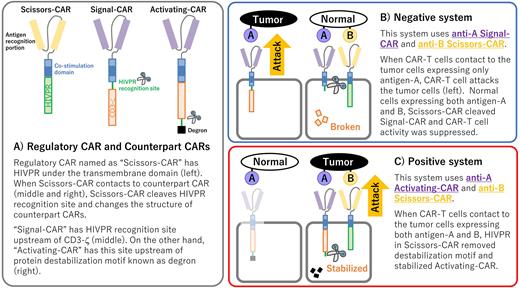
To improve target-cell-specificity of CAR-T cell, we established a protease-mediated regulatory CAR, named "Scissors-CAR" (panel A). This regulatory CAR is constituted with extracellular antigen recognition portion and cytoplasmic Human Immunodeficiency Virus protease (HIVPR) catalytic domain. When CAR-T cells expressing both anti-A Scissors-CAR and anti-B Counterpart-CAR that has HIVPR recognition polypeptide contact with target tumor expressing both antigen-A and B, these two CARs interact on cell membrane. This interaction induces Scissors-CAR-mediated cleavage of target polypeptide and structural change of Counterpart-CAR. Previously, we reported double antigen recognition system using Scissors-CAR. Previous Counterpart-CAR named "Signal-CAR" had HIVPR recognition polypeptide upstream of CD3-zeta, so Scissors-CAR-mediated cleavage broke the CAR protein structure and suppressed CAR-T cell activity (panel B, named "Negative system"). This system allowed CAR-T cells to directional attack against target cells expressing only antigen-A and protect the cells expressing both antigen-A and B.
Here, we established another double-antigen recognition system using Scissors-CAR. In many cases including both hematologic malignancies and solid tumors, additional ectopic antigens are expressed on malignant cells. If CAR-T cells empowered to directional attack against target cells expressing both antigen-A and B, much more tumors can be treated with this tumor immunotherapy (panel C, named "Positive system"). To establish this directional CAR-T cells, we constructed novel Counterpart-CAR named as "Activating-CAR" constituted with target recognition portion, co-stimulatory domain, CD3-zeta, and protein destabilization motif known as degron. Between CD3-zeta and degron motif, HIVPR recognition polypeptide was inserted. In vitro analysis revealed that this degron motif destabilized Activating-CAR and HIVPR-mediated cleavage and that release of degron motif increased Activating-CAR stability. Furthermore, cell proliferation of Jurkat cells expressing both anti-CD19 Activating-CAR and anti-HER2 Scissors-CAR was enhanced co-cultivated with target cells expressing both CD19 and HER2.
From these results, combined use of Activating-CAR and Scissors-CAR allowed CAR-T cells to directionally activate against target cells expressing two antigens recognized by each CAR. By choosing the Counterpart-CAR from "Signal-CAR" or "Activating-CAR", this protease-mediated double antigen recognition system could optimize target-cell-specificity of CAR-T cells for the individual tumor and contribute to expand the target tumors of CAR-T cell therapy.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal